COLOWELL Noninvasive ColorectalCancer Screening Test
SFRP2+SDC2
COLOWELL uses a patented double-S-target bowel cancer testto predict colorectal cancer risk by combining todetect themethylation levels of the SFRP2 and SDC2 genes in shed cells ina stool sample based on a large-sample data analysis model.
- 92.2%Clinical Sensitivity
- 91.9%Clinical Specificity
- 62.9%High-Grade Intraepithelia[ Neoplasia (HIN)

Global Colorectal Cancer 1.93 million new cases Ranked No. 3

Colorectal cancer is not scary
early detection is key
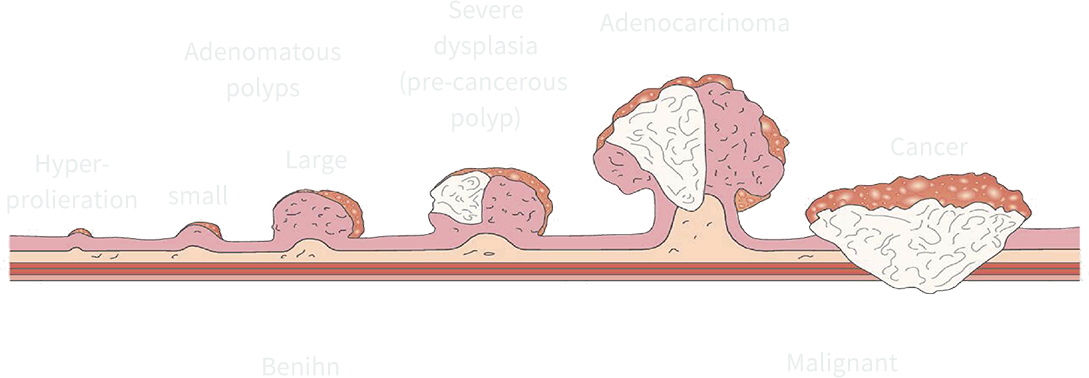
The slow onset of colorectal cancer, from precancer-ous polyps to cancer, takes 10-15 years. This biological feature makes colorectal cancer suitable for ear-lydetection. Colorectal cancer can be effectively pre-vented by detecting and removing precancerous le-sions such as adenomas.
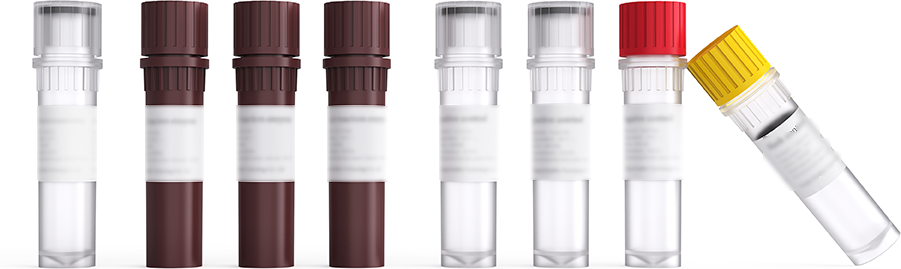
One-stop localization solution


The COLOWELLone-stop localization solution covers fecal sampling devicenucleic acid extraction kit, nucleic acid purification kit and human SFRP2 andSDC2gene combined methylation detection kit. The whole-process profession -alquality control, digitalization and traceability can assist the clinic to achieveamore convenient and accurate diagnosis of colorectal cancer
Six Advantages of COLOWELLO
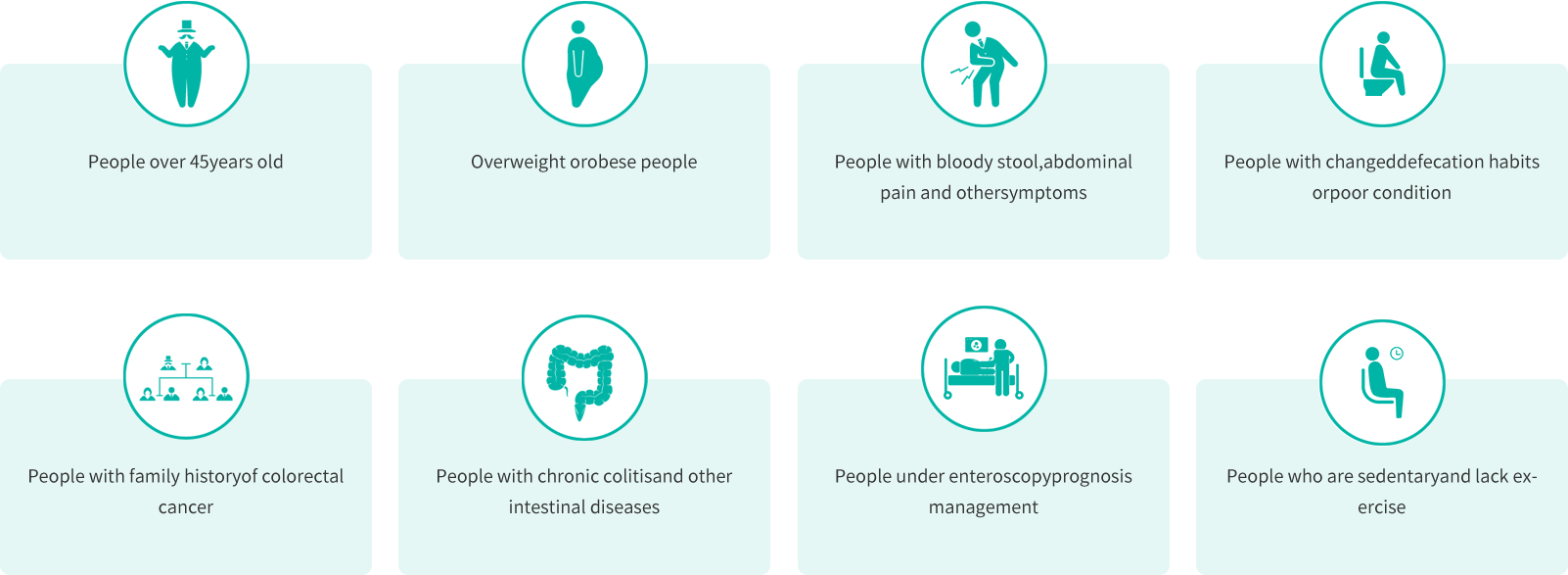
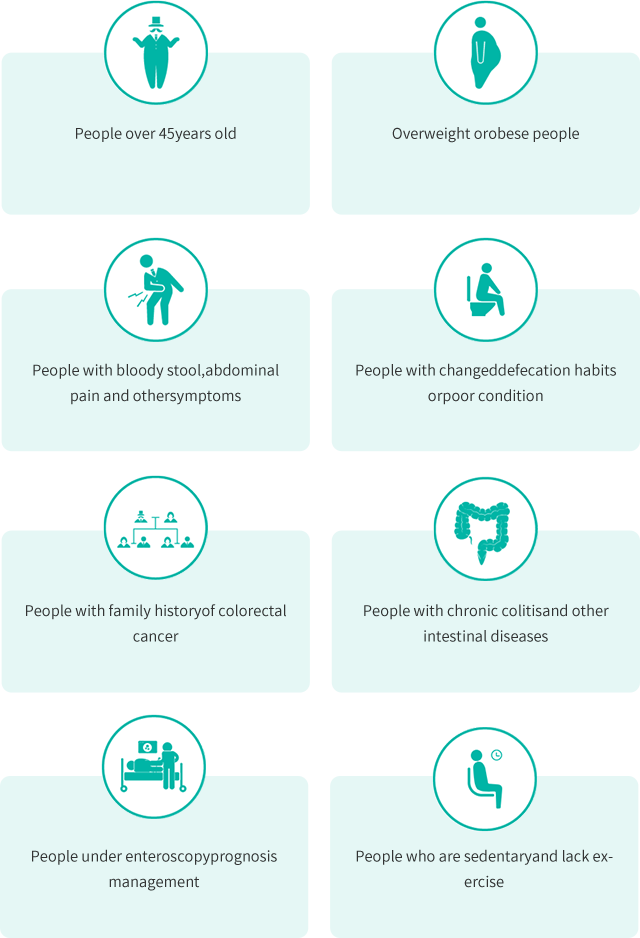
Suitable Population
Certificate

- Patented invention
ZL201810884870.8

- Medical Device Registration Certificate
(in Vitro Diagnostic reagents)
Nationalinstrument registration
20223400637

- Eu CE certification
NL-CA002-2022-67703





